Are GHG emissions and air pollution correlated?
With a couple of exceptions, countries with the highest cumulative GHG emissions from 1970-2021 have the cleanest air, while those with the lowest cumulative GHG emissions have the dirtiest air.
Primary air pollutants…
The primary air pollutants are a group of substances released into the atmosphere as a result of natural processes and various human activities. These pollutants have detrimental effects on air quality and can have adverse impacts on human health, ecosystems, and the environment. Here are the primary air pollutants:
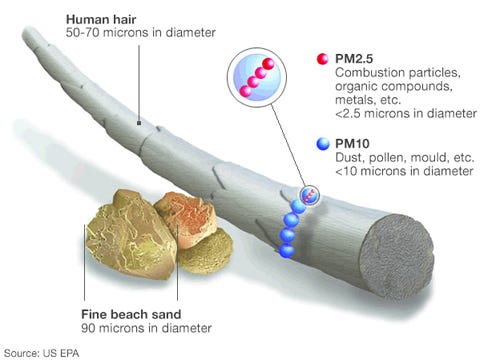
Particulate Matter (PM): Particulate matter refers to tiny solid or liquid particles suspended in the air. These particles can vary in size, and they are classified as PM10 (with a diameter of 10 micrometers or less) or PM2.5 (with a diameter of 2.5 micrometers or less). PM can originate from dust, pollen, combustion processes, industrial emissions, and other sources.
Nitrogen Oxides (NOx): Nitrogen oxides are a group of gases composed mainly of nitrogen dioxide (NO2) and nitric oxide (NO). They are produced naturally during the nitrogen cycle, which is the natural circulation of nitrogen among the atmosphere, plants, animals, and microorganisms that live in soil and water, as well as the combustion of fossil fuels, vehicle emissions, and industrial processes. NOx contributes to the formation of smog and acid rain and can have adverse effects on human respiratory health.
Sulfur Dioxide (SO2): Sulfur dioxide is a gas released from natural sources such as volcanoes, fires, and phytoplankton, as well as from the burning of fossil fuels, particularly coal and oil containing sulfur compounds. It is also emitted by industrial processes such as metal smelting. SO2 contributes to the formation of acid rain and can irritate the respiratory system.
Carbon Monoxide (CO): Carbon monoxide is a colorless and odorless gas produced by the incomplete combustion of fossil fuels and biomass and is also released into the atmosphere by volcanoes as they erupt, from the smoke of forest fires, from the natural gases in coal mines, and even from lightning. It is primarily emitted by vehicles, industrial processes, and residential heating. CO is toxic and can be harmful when inhaled in high concentrations, as it reduces the oxygen-carrying capacity of the blood.
Volatile Organic Compounds (VOCs): Volatile organic compounds are a group of carbon-based chemicals that easily evaporate at room temperature. They are released by various sources, including the burning of fossil fuels, industrial processes, the use of solvents and chemicals, forest fires, volcanoes, and plants. VOCs contribute to the formation of ground-level ozone (a component of smog) and can have adverse health effects.
Lead (Pb): Lead is a heavy metal that was once commonly used in gasoline, paints, and other industrial applications. While leaded gasoline has been phased out in many countries, lead can still be released into the air through industrial processes such as metal smelting. Lead is a toxic substance that can cause neurological and developmental issues, particularly in children.
These primary air pollutants can interact with sunlight, and other pollutants, leading to the formation of secondary pollutants such as ground-level ozone, sulfuric acid, and nitric acid. The control and reduction of these air pollutants are essential to improve air quality and protect human health and the environment.
Note: CO2 is not a pollutant.
The classification of carbon dioxide (CO2) as a pollutant depends on the context in which it is presented. From a strictly scientific and atmospheric perspective, CO2 is not considered a pollutant. It is a naturally occurring component of the Earth's atmosphere and plays a vital role in the Earth's carbon cycle.
However, in the context of climate alarmism, CO2 is often referred to as a pollutant due to its concentration increases due in some part to anthropogenic (human-caused) activities. The release of CO2 from human activities, such as the combustion of fossil fuels for energy production and transportation, is partially responsible for the increase in atmospheric CO2 levels. This increase is commonly claimed to be the key driver of climate change and global warming.
In regulatory and policy discussions, CO2 is often labelled as a pollutant such that it can be regulated and often taxed.
Air pollution from the combustion of fossil fuels…
The combustion of fossil fuels releases a complex mixture of particulates and gases into the atmosphere.
Particulate matter (PM) is a significant byproduct of fossil fuel combustion. It consists of tiny solid or liquid particles suspended in the air. These particles can vary in size and composition, ranging from coarse particles (PM10) to fine particles (PM2.5) and ultrafine particles. PM is composed of various substances, including soot, dust, ash, and chemical compounds. The specific composition depends on the type of fuel burned and the combustion conditions. For example, coal combustion tends to produce more sooty particles, while oil combustion can result in a mix of organic and inorganic compounds.
Gaseous pollutants are also released during the combustion of fossil fuels. Carbon monoxide (CO), a colorless and odorless gas, is produced when fuel does not burn completely.
Nitrogen oxides (NOx) are another group of gases released during fossil fuel combustion. They are primarily composed of nitrogen dioxide (NO2) and nitric oxide (NO). NOx emissions occur due to high-temperature combustion processes, such as those in power plants, industrial facilities, and vehicles. These gases play a significant role in the formation of smog, and contribute to the production of secondary pollutants like ground-level ozone.
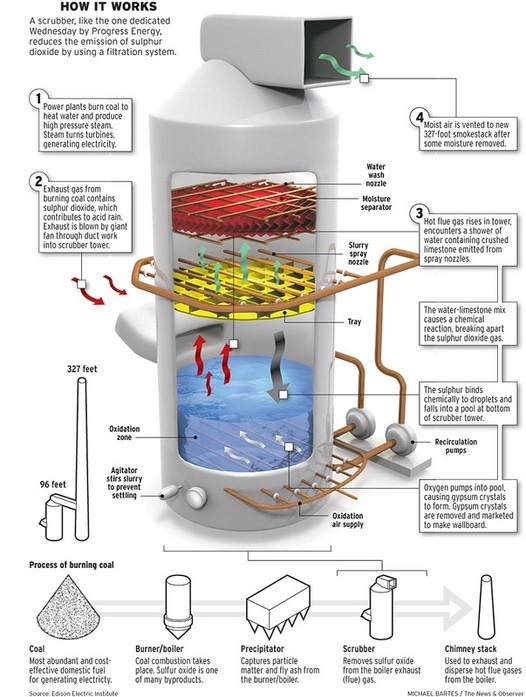
Sulfur dioxide (SO2) is a gas generated when fossil fuels containing sulfur compounds are burned. When released into the atmosphere, SO2 can react with water vapor and other pollutants, forming sulfuric acid (H2SO4) and contributing to the formation of acid rain.
In addition to these pollutants, fossil fuel combustion releases volatile organic compounds (VOCs) into the atmosphere. They arise from the incomplete combustion of fossil fuels, evaporative emissions from fuels and solvents, and industrial processes. VOCs react with other pollutants and sunlight to form ground-level ozone (O3) and secondary organic aerosols.
The exact composition and quantity of pollutants emitted depend on several factors, including the type of fossil fuel, combustion technology, and the presence of emission control measures.
So how do GHG emissions correlate with air pollution?
Keep reading with a 7-day free trial
Subscribe to Irrational Fear to keep reading this post and get 7 days of free access to the full post archives.