How do scientists estimate past temperatures from ice cores?
Oxygen isotopic analysis as a proxy for temperature.
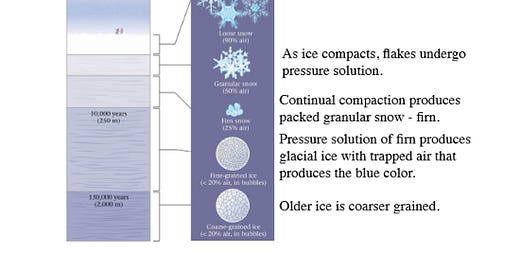
Glacial ice formation…
Glacial ice forms through the accumulation and compression of snow over long periods of time. It begins with the transformation of snow into firn, a granular, partially compacted snow. Over time, additional layers of snow accumulate on top of the firn, and the weight of the overlying snow compresses the underlying layers. With cont…
Keep reading with a 7-day free trial
Subscribe to Irrational Fear to keep reading this post and get 7 days of free access to the full post archives.