Nearly half the world's population relies on synthetic fertilizers made from fossil fuels.
Let's talk about the Haber-Bosch process.
What is the Haber-Bosch process?
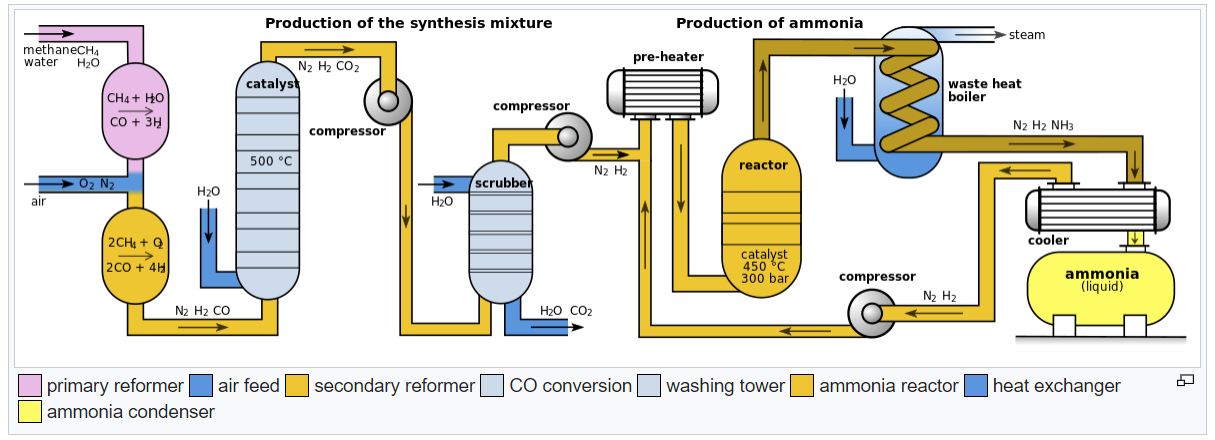
The Haber-Bosch process, also known as the Haber process, is a chemical process that converts nitrogen gas (N₂) from the air and hydrogen gas (H₂) from natural gas or other hydrocarbon sources into ammonia (NH₃). It was developed by Fritz Haber and Carl Bosch in the early 20th century and is one of the most important industrial processes in the world.
The process takes place at high pressures and temperatures, typically around 200-300 atmospheres and 400-500 degrees Celsius. These conditions are necessary because the reaction between nitrogen and hydrogen is not spontaneous under normal conditions. Instead, it requires the application of a catalyst, typically composed of iron, promoted with small amounts of other elements such as aluminum oxide, calcium oxide, or potassium oxide.
The overall reaction of the Haber-Bosch process is:
N₂ + 3H₂ ⇌ 2NH₃
The process involves a series of reactions and steps. First, the nitrogen and hydrogen gases are compressed and mixed in a ratio of three parts hydrogen to one part nitrogen. Then, they are heated to the reaction temperature and passed over the catalyst bed. The catalyst provides a surface for the reaction to occur, facilitating the breaking of the strong triple bond in the nitrogen molecule and the formation of ammonia molecules.
The reaction is exothermic, meaning it releases heat. Therefore, the process must be carefully controlled to maintain the desired temperature. The ammonia gas produced is then cooled and condensed into a liquid, which is stored for further use or transported.
The Haber-Bosch process revolutionized the production of ammonia and played a crucial role in the development of modern agriculture. Ammonia is a key component of fertilizers, providing plants with the necessary nitrogen to grow. It has greatly increased crop yields and has had a significant impact on global food production.
The process also has important industrial applications beyond agriculture. Ammonia is used in the production of various chemicals, such as nitric acid and urea, and is an essential ingredient in the manufacture of explosives, plastics, and synthetic fibers.
How much of the world’s population relies on synthetic fertilizers?
Keep reading with a 7-day free trial
Subscribe to Irrational Fear to keep reading this post and get 7 days of free access to the full post archives.